Nano metals have poor stability due to the introduction of a large number of grain boundaries. Generally speaking, the growth temperature of nanocrystalline grains is much lower than the recrystallization temperature of coarse crystals, and some nanocrystalline pure metals grow up even at room temperature. Poor stability has become a significant bottleneck restricting the preparation and application of nanometals. The traditional method of stabilizing nanocrystals is mainly to reduce the interfacial energy or drag the grain boundary migration through alloying.
In 2018, nanometal scientists from the Chinese Academy of Sciences discovered the abnormal grain size effect of thermal stability of nanocrystals in nanocrystalline pure copper and pure aluminum prepared by plastic deformation, that is, smaller than the critical size. The deformation mechanism changes from dominated by dislocations to governed by incomplete fractures, the grain boundary relaxation mechanism is activated, and the stability of nanocrystals does not decrease but rises. Subsequently, they found that, despite the difference in the internal mechanism of grain boundary migration under heating conditions, this abnormal grain size effect also exists in the mechanical stability of nanocrystals under stress.
It is understood that in some nanometals, such as pure copper, nano-grains grow even at room temperature. This inherent instability brings difficulties to the preparation of nano-metallic materials; on the other hand, it also limits the practical application of nanometals.
The study also found that the abnormal stability of nanocrystals not only occurs in low- and mid-level fault energy metals such as pure copper but also in high-level fault-energy pure nickel. The discovery of ultra-high stability nanocrystals is not only significant for us to understand the deformation mechanism of nanocrystals and the behavior of grain boundaries at nanometer size but also shows the possibility of developing nanocrystals used at high temperatures.
However, the grain size of the pure metals prepared by the currently commonly used severe plastic deformation methods (such as equal channel extrusion, lap rolling, etc.) is usually in the submicron scale, and it is challenging to initiate the grain boundary relaxation mechanism during processing. For example, the grain size of pure copper prepared by severe plastic deformation is mostly in the range of 100-200 nm, and its stability is poor. Recently, researchers have discovered that rapid temperature increase can introduce annealing twins into nanocrystalline copper, thereby achieving "thermal relaxation" of the nanocrystalline grain boundaries and improving the thermal stability of the nanocrystals. One of the difficulties faced by the introduction of annealing twins in nanocrystalline copper is that unstable nanocrystals have a grain growth stability of only 393 K, which is far below the temperature at which annealing twins are generated (——523 K) During the heating process, twins are not produced, and the grains have grown up. Based on the Kissinger effect, the researchers proposed that increasing the heating rate can increase the grain growth temperature without affecting the twinning growth temperature. Therefore, rapid temperature rise not only avoids grain growth but also produces growth twins. The pure Cu with a grain size of about 80 nm was rapidly heated to 523 K at a rate of 160 K / min for 15 minutes and then cooled. The grain size of the material did not change significantly, but the number of twins increased significantly. Like deformed twins, these grown twins can also relax the grain boundaries and enhance the thermal stability of the nanocrystals. After heat treatment, the apparent growth temperature of the nanocrystals increased from below 393 K to above 773 K.
The thermal relaxation method of rapid temperature increase to improve the stability of nanocrystals can be used to enhance the security of submicron and nanocrystals obtained by general severe plastic deformation and is of great significance for the development of highly stable nanomaterials and the promotion of nanometal applications.
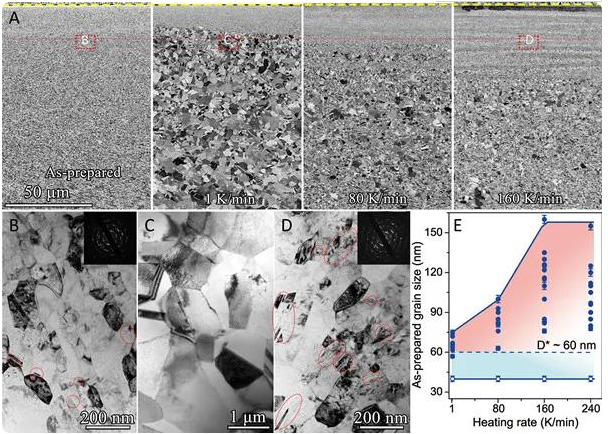
Figure 1. Nanocrystalline copper stabilized by rapid heating. (A) Typical cross-sectional scanning electron micrographs of the surface gradient nanostructures of the as-prepared samples and samples heated to 523K at different heating rates (1, 80, 160K / min), respectively. (BD) Preparation samples and samples after heat treatment at different heating rates from the surface-typical transmission photos of nanocrystals at a depth of 25 μm (corresponding depth is shown in the dotted frame in Figure A, the average grain size of the prepared samples at this depth About 80nm). (E) The range of grain size corresponding to the surface stabilized nanocrystals in the example after heating to 523K at different heating rates. The reliable and hollow dots in the figure, respectively, represent the grain size corresponding to the upper and lower boundaries of the stable nanocrystalline layer observed in the experiment. The dotted line represents the upper limit of the average grain size of the stable nanocrystals during the 523K heat treatment of the prepared sample (D * —— 60nm ), The error bar refers to the standard statistical error of the average grain size.
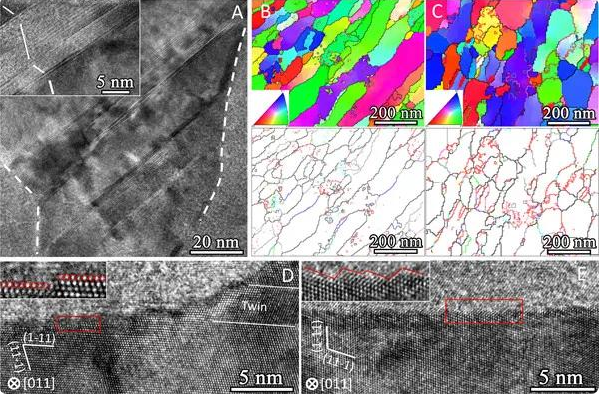
Figure 2. After rapid heating, a large number of twins are formed inside the nanocrystals, and the grain boundaries are planarized. (A) A typical high-resolution photo of nanocrystals in the area shown in Figure 1D. The inset in the upper left corner is an atomic image of the nano twins in the grain. (B, C) Typical grain boundary character distribution in the sample after preparation and heating to 523K at a heating rate of 160K / min. Different colors represent different types of grain boundaries, red is the twin grain boundary, gray is the small-angle grain boundary, black is the significant conventional angle grain boundary, and other colors are other individual bulky lattice interfaces (Σ <29). (D, E) High-resolution photos of typical grain boundaries in the area shown in Figure 1D. The illustration in the upper left corner is an enlarged view of the solid red frame.
If you were given a chance, what items would you use 3D printing for? Is it a toy bag or earring jewelry? In fact, behind every 3D print original product being presented, we must rely on 3D printing technology and equipment to complete.
Ten years have passed since the concept of 3D printing was proposed. From the birth of 3D printing technology in 1986 to the present, after more than 30 years of technology accumulation, two technical genres of metal 3D printing and non-metal 3D printing have been formed globally. The debate about the merits of metal 3D printing and non-metallic 3D printing has also made 3D printing this "quiet lake" rippling.

Most 3D printing manufacturers currently choose SLA and SLM technology paths, and SLM is particularly hot soon. SLM stands for Selective Laser Melting, which is a technology that directly heats metal powder through a laser to melt it through cooling molding completely. Generally speaking, SLM's 3D printing technology is injecting powerful product remodeling capabilities into industrial manufacturing from aspects such as shortening production time, improving functionality, reducing the number of parts, improving design freedom, and improving supply chain management efficiency.
In the metal 3D printing process, the quality of the metal powder is one of the critical factors that affect the structure and performance of the final printed parts. At present, improving the performance of metal powders and product materials has become an essential issue for the industry. After all, the better the quality of the metal powder and the smaller the particle size, the better the compactness and mechanical properties of the printed product.
In a comprehensive comparison, the advantages of non-metallic 3D printing are mainly customizable and moldless, but material properties limit it. It is used for the production of molds and samples, and it is difficult to expand the quantity and price; In addition to the advantages of moldless and customizable, the printing efficiency, printing quality, and printing accuracy are more significantly improved than traditional metal processing techniques. It is worth noting that metal 3D printing can complete the printing of high-complexity and high-precision parts that cannot be made by traditional crafts and has excellent potential for development.
In the automotive field, 3D printing has a vast application space, and the development prospect is promising. Some automobile companies have also begun to use 3D printing technology for interior manufacturing and parts customization under the trend of lightweight and intelligent vehicle manufacturing. Since 1991, BMW has incorporated 3D printed parts into the concept car development system, which shows its forward-looking vision. In the past ten years, BMW has produced 1 million pieces using 3D printing technology, and its output has reached a high level in the automotive industry.
From another perspective, whether it is automobile tires or aerospace, 3D printing technology is inseparable from the development of three critical factors, namely equipment, materials, and technology. The launch of a high-quality product requires not only excellent performance materials but also innovative processes and high stability 3D printers. The spread of non-metallic 3D printing and 3D printing in various fields needs to pay attention to the relationship between technology, equipment, and materials.
From metal printing to non-metal printing, from titanium alloys, 316 stainless steel to non-metallic materials, from bookcases and chairs, cartoons, to aerospace precision parts, highly complex components, the products that can be manufactured by 3D printing The more precise and diverse it becomes. Metal 3D printing and non-metallic 3D printing are like twin brothers, with similarities and differences. In each subdivision scenario, both technologies will continue to "light up" to illuminate the world for industrial upgrading.
According to market research institutions, the 3D printing market will reach USD 55 billion by 2029, which is consistent with the 3D printing market's goal of accounting for approximately 2% of the global manufacturing industry's market value of USD 12 trillion in 2030. Such a vast market space has attracted the attention of many investors. It is easy to find that the pioneers in the industry are jointly painting a brilliant new picture of the 3D printing industry. In the next few years, to better meet the actual needs of users in various industries, the speed of 3D printing technology will be further accelerated, and new application models will blossom.
According to reports, researchers from Texas A & M University and AFR have developed a technology for defect-free metal 3D printing of martensitic steel parts. Compared with similar steels, martensitic steels are more reliable and more cost-effective and are used in aerospace, automotive, and defense industries.
Although sturdy steel is widely used, it is usually expensive. The only exception is martensitic steel, which costs less than one dollar per pound and has a relatively low cost. They have also developed a 3D printing framework that can print these hard steels into flawless objects of any geometry.
What is martensitic steel?
For thousands of years, metallurgists have been carefully adjusting the composition of the steel to enhance its performance. To this day, there is a product called martensitic steel that stands out in its steel category because of its higher strength and more cost-effectiveness.
Steel is a material smelted from iron and carbon. Martensitic steel is manufactured by high-temperature quenching. This sudden cooling process restricts the carbon atoms to the iron crystals, giving martensitic steel its unique strength.
△ Martensitic steel powder for 3D printing. The picture shows an enlarged view of the steel powder.
The industry has a high demand for hardened steel, but its price is too high. An exception is a martensitic steel, which has a relatively low cost, less than one dollar per pound.
Martensitic steel is very suitable for the fields that need to manufacture high-strength, light-weight parts without increasing costs, such as aerospace, automotive, and defense industries.
Technology improvement 3D printing high strength defect-free martensitic steel
To have multiple uses, it is necessary to assemble martensitic steel, especially a type called low-alloy martensitic steel, into objects having different shapes and sizes according to specific applications. Additive manufacturing (commonly referred to as 3D printing) provides a practical solution. Using this technology, a single layer of metal powder can be heated and melted in a pattern by using a high-energy laser beam to build complex parts layer by layer. Connect and stack all these layers to print out the final 3D printed object.
However, 3D printing of martensitic steel using lasers can cause defects in the form of pores in the material.
To solve this problem, the research team had to start from scratch and find out the laser settings that could suppress such defects.
In the experiment, first, an existing mathematical model was used to predict the melting of a single layer of martensitic steel powder under different laser settings. Next, by comparing the observed defect types, number, and model predictions, they improved the printing framework. After many iterations, their structure can make predictions more accurately. The researchers said that this method does not require additional experiments, which saves more time and effort.

△ The US Air Force Research Base conducted studies on the printed samples such as porosity, mechanical strength, and impact toughness, and the display's mechanical properties were excellent.
Although the initially developed process was for martensitic steel, they have made the technology versatile enough so that the same 3D printed pipeline can also be used to build complex objects from other metals and alloys.
This is an essential development for all types of metal additive manufacturing industries. Whether it is a simple part like a screw or a more intricate part of turbines such as landing gear or a gearbox, it will become more precise in the future To meet the needs of different industries.
This innovative prediction technology will shorten the time to evaluate and find the appropriate printing parameters for martensitic steel. However, testing the possibility of all laser settings and evaluating which may cause defects is very time-consuming and sometimes impossible. Yet, a quick and straightforward step-by-step process was developed by combining experiments and modeling to determine which setting is most suitable for 3D printing martensitic steel.
Graphite, also known as multilayer graphene, is made up of layers of carbon atoms, each just one bit thick, and its structure determines its superior properties. When graphite sheets are reduced to only a few layers of graphene, essential features such as the total surface area per unit mass of the material and the mechanical properties of the sheet are greatly improved. In other words, graphene is more than just a thin layer of graphite. Unfortunately, many graphene manufacturers don't seem to know the difference or don't care. Kaling et al. .published a systematic study of graphene sold by 60 manufacturers in the journal advanced materials ,and found that many expensive graphene products were fakes made from graphite powder.What would the world be like if everyone could sell antibiotics without following quality standards and laws? Not only are the potential side effects worrisome, but the efficacy is questionable, and even potentially deadly, so many people are deterred from taking antibiotics. Emerging nanomaterials like graphene may not be dangerous, but the lack of industry standards has had equally unacceptable consequences.
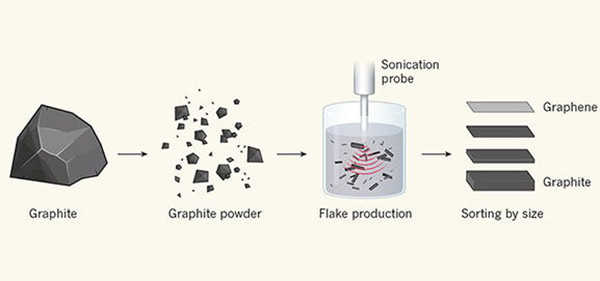
One of the most sophisticated methods for the preparation of commercial graphene is the liquid phase exfoliation method, in which graphite is first ground into a powder and then mechanically separated into beautiful graphite flake layers in a solvent. These precious flaps contain a few layers of graphene and are then sifted, as shown in figure 1. The graphene produced by this method can be widely used in battery technology, composite materials, and solar cells. The preparation of graphene by liquid-phase stripping was initially achieved by the process of acoustic degradation of graphene sheets. Later, studies have shown that even kitchen mixers can produce violent turbulence, allowing the separation of graphene layers without damaging them.

Most commercial graphene blocks are made by grinding a stone mill into a powder, then mechanically forcing the graphite powder into tiny flakes in a solvent using methods such as acoustic degradation. The size of the graphite sheet is not shown. Then according to the size and thickness of the graphite sheet layer screening. Kaling et al. 1 analyzed commercial graphene produced by 60 manufacturers and found that most samples contained less than 10% graphene (graphite sheet 5 with fewer than ten layers of carbon atoms). The rest of the examples are necessarily just graphite powder.

But what does the graphene sheet have to be thin to be graphene? It is generally accepted -- and endorsed by ISO, the international organization for standardization -- that if a graphene sheet contains more than ten layers of graphene, it is essentially graphite. Kaling et al. argue that this seemingly arbitrary threshold setting has a physical basis. For example, thermodynamics holds that in graphite sheets with ten or fewer layers of carbon atoms, each carbon atom can maintain the crystalline structure of graphene at room temperature. Furthermore, the hardness of the graphite sheet is proportional to the cube of the thickness of the single layer, which means that the elasticity of the thin graphene sheet is several orders of magnitude higher than that of the thick graphite sheet.

So size matters a lot: in practice, graphene and graphite powder can have very different effects. If there are no clear criteria to identify the quality of commercial graphene and graphite powder is falsely advertised as expensive, and high-quality graphene, companies, and researchers risk wasting time and money on research. This will hinder the development of graphene technology and harm the manufacturers and developers who make graphene seriously.
But are such fears misplaced? In a study that is designed to answer that question, Kaling et al. built on a well-established set of graphene identification methods to establish a systematic test protocol that was used to benchmark 60 graphene products manufactured by different vendors. The research has unnerved many businesses. The results show that there are remarkable differences in the distribution of critical indicators such as material size, structural integrity, and graphene purity. Surprisingly, less than 10 percent of the samples of the majority of products containing graphene contained graphene, a sheet of graphite with fewer than ten layers of carbon atoms. None of the products tested contained more than 50 percent graphene, and most were heavily contaminated, most likely because of chemical contamination during processing.
The high-profile scientific advances, technological breakthroughs, and considerable investments in graphene appear to have opened up a new world for commercial speculators: the study suggests that some manufacturers even price graphene as a black powder whose main ingredient is cheap graphite.
The problem is exacerbated by the unusually low barriers to entry to become a supplier of graphene -- anyone can buy a chunk of graphite, grind it into a powder and then develop a sales website to make money. If you don't introduce accepted standards and testing protocols, you risk further errors. Many of the emerging graphene applications are closely related to socially relevant issues such as health, climate, renewable energy, and sustainable development. If you start with fake graphene, some areas may not make any progress at all.
So Kaling and his colleagues' paper is a necessary wake-up call for graphene manufacturers, buyers, and researchers to agree that everyone benefits from a transparent market for graphene, except for vendors who ignore moral decency and to adhere to strict standards. The ISO graphene dictionary, a document detailing standard terms for graphene, and the national physical laboratory's practical guide to graphene identification have made a good start. Standardization of the graphene market should now continue.
It is worth noting that Kaling et al. did not cover all the large blocks of graphene on the market. And, although they analyzed a large number of products prepared by liquid-phase stripping, the criteria they used to screen them may have been biased, though they have tried to rule that out. They may also have inadvertently missed some of the high-quality graphene made by the best manufacturers. Moreover, they note that graphene is often selected for different properties for different application purposes, making it more difficult to unify quality metrics.
Still, the study is a timely and successful example of the kind of rigorous thinking needed for rapid technological change. Not just for graphene, but for any nanomaterial research that comes to market. Frankly, once there is no quality control, there is no quality at all.
Cadmium telluride thin-film solar cells are much cheaper to produce than crystalline silicon and other materials used in solar cell technology. Standard process, low energy consumption, after the end of the life cycle, recyclable, strong and weak light can be generated, the higher the temperature, the better the performance. With these advantages, CdTe thin-film Solar cells have begun to challenge conventional crystalline silicon Solar cells in the global market share. However, cadmium telluride solar cells still have some drawbacks of their own.

Tellurium is a rare element on the earth. The primary problem facing the development of cadmium telluride thin-film solar cells is whether the storage of tellurium on the planet can meet the requirements of industrial-scale production and application of cadmium telluride solar cell modules. Tellurium used to be in the form of copper, lead, zinc, and other mine byproducts, such as slag, as well as waste from smelters such as anode slime.
Although tens of thousands of tons of tellurium are known to exist on earth, and 130 to 140 kilograms of tellurium can be used to produce 1MW cadmium telluride thin-film solar cells, according to reports, they are nowhere near as abundant as silicon. Cadmium telluride thin-film solar cells contain the heavy metal element cadmium. Many people are concerned about the environmental impact of the production and use of cadmium telluride solar cells. That's why some companies and experts have been reluctant to step into the development and production of cadmium telluride solar cells for years.
To that end, scientists at Bookham national laboratory in the United States studied this question. They systematically studied the emissions of heavy metals from crystalline silicon solar cells and cadmium telluride solar cells per unit of electricity generated from conventional sources such as coal, oil, and natural gas and nuclear power. In the analysis of solar cells, the processing of raw ore into the materials needed for solar cells, the preparation of solar cells, the use of solar cells, and other life-cycle processes are considered. The results showed that petroleum had the highest cadmium emissions, at 44.3g/GWh, followed by coal at 3.7g/GWh. The discharge of solar cells is less than 1g/GWh, and the lowest emission of cadmium telluride is 0.3 g/GWh. Like natural gas, silicon solar cells emit about twice as much cadmium as cadmium telluride solar cells.
They also studied emissions of other heavy metals in the production and use of silicon solar cells and cadmium telluride solar cells. The results showed that cadmium telluride solar cells also emitted less arsenic, chromium, lead, mercury, nickel, and other heavy metals than silicon solar cells. The conclusions of this study are based on a systematic investigation of the cadmium telluride thin-film Solar cell production line and the on-site use of cadmium telluride Solar cell modules by First Solar, as well as an analysis of the technology and the use environment of other Solar cells and energy production enterprises. The scientific and impartial research results have been recognized at home and abroad. The researchers' presentation at the 2006 European materials conference on solar materials for sulfide semiconductors attracted substantial attention from the participants.

U.S. researchers have also studied cadmium contamination in thin-film solar cell modules that are damaged by accidents such as fires. They tested the cadmium telluride thin-film solar cell modules in a double-glass package at temperatures up to 1100 ° c, simulating a fire in a building. The results showed that the glass softened to the point of melting at high temperatures, the compound semiconductor film was encased in the softened glass, and the cadmium loss was less than 0.04 percent of the total cadmium content of the battery. Taking into account the risk of fire, cadmium emissions during use are less than 0.06mg/GWh.
Although experiments have shown that cadmium telluride thin-film solar cell modules are safe for use, establishing a mechanism for recovering end-of-life battery modules and damaged modules could boost public confidence. The separated Cd, Te, and other useful materials can also be used to manufacture the relevant documents for the production of solar cell modules for recycling. Studies in the United States and Europe show that it is technically feasible and that the benefit of recycling materials is higher than the cost of recycling. First Solar's cadmium telluride Solar modules are sold under recycling contracts paid for by the plant.
To sum up, cadmium telluride solar cells are environmentally friendly in production and use.

The physical properties of the 16 groups of elements in the periodic table change rapidly from top to bottom. Oxygen, of course, is a gas; polonium is a metal, but the ingredients in between, sulfur, selenium, and tellurium, are reliable, with their metallic properties enhanced in turn. I think tellurium (Te) is the most exciting element in this group, and its features are remarkably similar to those of most metals.

Tellurium is a rare metal. The amount in the earth's crust is about 1 PPB (one in a billion), more unique than gold, platinum, or other so-called "rare earth" elements. So why should we care about something so obscure? Borrow a phrase that George Mallory used to explain why he climbed Mount Everest, and one reason was "because it exists."
However, it is now wise to please the funders, so other reasons include meeting the essential industrial applications and developing more application potential. For realizing these applications, tellurium must satisfy industrial production as well as basic laboratory research, which brings us to the origin of tellurium.
Because tellurium is so rare and dispersed, it is not profitable to mine it. Tellurium, however, is often found in nature along with gold, silver, copper, and other valuable metals. Gold tellurides are the most common gold-bearing minerals. Tellurium, by the way, was discovered in 1782 by Austrian mineralogist Franz Josef Muller von Reichenstein in a gold mine in Hungary. Tellurium is, therefore, a by-product of the refining of the metals; The primary source is anode slime in the electrolysis of refined copper. The tellurium content in the mud averaged about two wt%. World production of tellurium has increased dramatically in recent years, reaching 500 tonnes in 2007. Moreover, the price of tellurium has been rising, as has demand.
The synthesis, structure, and properties of tellurium - containing compounds have attracted much attention in recent years. We can take tellurium compounds whose compositions have been characterized by X-ray diffraction as a reference. In the past several decades, the number of inorganic tellurium compounds has increased by about 40 percent; Organic and metallic organotellurium compounds increased by nearly 70 percent. This is a surprising number since tellurium is not essential to life. Moreover, many of the syntheses and products of tellurium chemistry smell even scarer than their sulfur homologs!
Personally, when we started working on ternary solid-state tellurides 25 years ago, we didn't know if these compounds were similar to ternary selenides. Some are similar, and some are different. The difference even extends to binary mixtures. For example, Nate exists, and NaSe is not known. If you ask a first-year chemistry student to describe Nate's structure and oxidation state, the answer is undoubtedly the well-known tendency of sulfur, selenium, and tellurium to form chains or rings, especially tellurium, unlike oxygen.

Nate is Na6Te(Te5), which contains Na+ cations and Te2− and the z-shaped Te54− anion. The z-shaped anion has two Te-Te bond lengths, 2.82 and 3.08 A, which is the difference between tellurium and sulfur. Pyrite (also known as "fool's gold" because of its golden luster) is found in FeS2, where the sulfur atoms are 2.08a apart and are of s-s single bond length. In the form of iron, the oxidation state is +II instead of the +IV that one might mistake by its formula.
All stable sulfur compounds containing s-s bonds are linked by single bonds. In contrast, stable compounds containing Te−Te may contain bonds 3.6 A or longer but still smaller than van der Waals bonds (4.1a), in addition to the regular Te−Te single relationship (2.74a). This makes the identification of formal oxidation states impossible. Tellurium's unique allotrope is stable at room temperature and appears as an infinite spiral (pictured above).In this helical structure, te-te spacing is approximately 2.83a, but there is also A short spacing of 3.49a adjacent interatomic interaction. The bonding of tellurium-bearing solid-state compounds has led to many theoretical studies that are far more interesting than their lighter homologs.
Tellurium is one of the most crucial alloy additives in the metallurgical industry. Steel and copper added with tellurium are more comfortable to process and can also be used in cast iron to minimize thermal shock and thus reduce fatigue. Another important industrial application of tellurium is as an accelerator and vulcanizer in the rubber industry. These applications do not require high purity tellurium. However, the need for high purity tellurium is growing in some new and evolving demands in the electronics industry. Tellurium, for example, is used in newly developed phase-shift memory chips and rewritable CDS, DVDs, and Blu-ray discs.
Bismuth telluride is widely used in thermoelectric cooling equipment. These devices are commonly used in electronics and consumer products. Recently more and more bismuth telluride has been used in portable food coolers. Believe it or not, in car seat cooling systems. Tellurium's most significant impact on our lives is likely to come from cadmium telluride solar panels, which, while still in their infancy, are one of the most efficient generators. Tellurium may yet save humanity!
Lanthanum stearate is a new type of polyvinyl chloride (PVC) thermal stabilizer that replaces metal soap salts. The traditional method of synthesizing lanthanum stearate is a metathesis reaction method. The product obtained by this method contains inorganic salt impurities. To disperse stearic acid and sodium stearate, a large amount of water is used as a solvent, so the equipment is large, the energy consumption is high, and the water consumption is high. So is there a way to directly synthesize lanthanum stearate? This article will explore the feasibility of directly integrating lanthanum stearate. The oxide used in the experiment is lanthanum oxide (La2O3). To prevent the product from containing chloride ions, so as not to catalyze the decomposition of PVC, nitric acid was selected when the acid was dissolved. To overcome the disadvantage of stearic acid being insoluble in water, a small amount of ethanol was used as a solvent. Considering that many factors are affecting the synthesis process, to reduce the number of experiments and obtain the most suitable process conditions, an orthogonal experiment method is used.

Experimental part
1.Raw material
Stearic acid (HST), lanthanum oxide (La2O3), industrial products, solid alkali, nitric acid, ethanol, and ammonia are all chemically pure.
2. Experimental method
First, we need to prepare the lanthanum nitrate. Measure 1.63 g (0.05 mol) of La2O3 into a 200 mL beaker, add 8.33 mL of a 1: 3 nitric acid solution, and slowly heat in a fume hood until La2O3 dissolves and the answer is clear. Then dilute with distilled water to the specified concentration to obtain a lanthanum nitrate solution.
Next, we prepare the lanthanum stearate. The reaction formula for preparing lanthanum hard acid is: La (NO3) 3 + 3NH3 · H2O La (OH) 3 ↑ + 3NH4NO3 (1) 3C17H35COOH + La (OH) 3 (C17H35COO) 3La ↑ + 3H2O. Take a clean 500 mL three-necked flask, add 8.52 g (0.03 mol) of stearic acid and an appropriate amount of ethanol as a solvent, put it in a water bath, install the magnetic stirring and dropping funnel, and heat to the specified temperature. Start stirring to dissolve the stearic acid completely. Then add the specified concentration of lanthanum nitrate solution. After stirring well, slowly add the ammonia solution from the dropping funnel, and then keep it to the specified time, and filter while hot.
Finally, the lanthanum content of the product needs to be determined, and the xylenol orange decomposed by nitric acid is used as an indicator, and the EDTA titration analysis is used.

Factors affecting experimental results
1.Effect of reaction temperature on an experiment during the synthesis of lanthanum stearate
The reaction temperature is the most critical factor affecting the yield of rare earth. As the reaction temperature increases, the return of extraordinary earth increases. This is because as the temperature increases, the energy of the reactant molecules increases, the chance of effective collision increases, and the reaction are more complete. Because ethanol is used as the stearic acid solvent in this experiment, the boiling point of ethanol is 78.3 ℃ under normal pressure, so the reaction temperature of about 80 ℃ is appropriate. Excessive heat can easily cause rapid volatilization of ethanol, but the yield will decrease.
2.Effect of Lanthanum Nitrate Solution Concentration on Experiments in the Synthesis of Lanthanum Stearate
As the concentration of the lanthanum nitrate solution increased, the yield of lanthanum increased. When the level reached more than 2.4 mol / L, the yield decreased instead. It may be that the level of the material liquid is too high, which is not conducive to the uniform mixing of the reaction materials. Therefore, it is appropriate to select a lanthanum nitrate feed solution concentration of 2.4 mol / L.
3.Effect of Ammonia Concentration on Experiments during the Synthesis of Lanthanum Stearate
During the reaction, to keep the reaction system from causing excessive alkali and hydrolysis of lanthanum stearate, ammonia was added in a dropwise manner. Because the solvents of ammonia water and lanthanum nitrate are both water, after the water is added, it first forms La (OH) 3 with lanthanum nitrate. It then reacts with stearic acid to form lanthanum stearate. Increasing the concentration of ammonia water is conducive to the reaction. Still, when the level of ammonia water is too broad because ammonia is volatile, it will volatilize when the response is still available in the future, resulting in insufficient ammonia water and reducing the yield. Therefore, the ammonia concentration is preferably 0.8 mol / L.
4.Effect of Stearic Acid Concentration on Experiments during the Synthesis of Lanthanum Stearate
The yield of lanthanum increases with the increase of stearic acid concentration (that is, the decrease in ethanol consumption), which is conducive to cost reduction. However, when the amount of ethanol is small, the viscosity of the reactant increases, which is not conducive to the reaction. Stearic acid concentration should be controlled at about 1.0 mol / L.
5.Effect of reaction time on the experiment of the synthesis of lanthanum stearate
Since this reaction is an acid-base neutralization reaction, the speed is fast. To make the reaction system under weakly acidic conditions, the result is carried out by adding ammonia dropwise. The dropping time is preferably 30 minutes. However, the result did not wholly end after the ammonia was added dropwise, and there must be a holding time. The holding time should be selected as 30 min.
One of the most widely used additives currently in medicine and supplements is magnesium stearate. Such supplements are present in supplements sold on the market. But it is often called another name, such as "vegetable stearate" or "stereo acid", which is almost everywhere.
Although this additive is ubiquitous, magnesium stearate is also one of the most controversial ingredients in the supplement world. In some respects, it is similar to the controversy over vitamin B17, and there is controversy as to whether this additive is a poison or a cure for cancer. Most importantly: Like most fillers and bulk additives, magnesium stearate is not good for the human body at high doses, but it is definitely not as badly harmful as some products, because it can usually only be used in very small doses.

What is magnesium stearate?
Magnesium stearate is a magnesium salt of stearic acid. Basically, magnesium stearate contains two compounds of stearic acid and magnesium. Stearic acid is a saturated fatty acid in many foods, including animal and vegetable fats and oils. Cocoa and flaxseed are examples of foods that contain large amounts of stearic acid.
After magnesium stearate is broken down into components in the body, its fat is essentially the same as stearic acid. Some sources even claim that its magnesium portion can be used to provide essential minerals to the body. Given the widespread prevalence of magnesium deficiency, this suggests that magnesium stearate is actually good for the body.

Uses of magnesium stearate
Magnesium stearate is the most common ingredient used to form tablets because it is a magical lubricant. It's called a "flowing agent" and it helps speed up the manufacturing process because it prevents ingredients from sticking to mechanical equipment. A negligible amount is required to apply a powder mixture to almost any drug or supplement mixture.
Not only can magnesium stearate be transported smoothly on the machine that produces the pill, it also makes it easier to swallow the pill and move it down the gastrointestinal tract. Magnesium stearate is also a common excipient, which means that it helps to enhance the therapeutic effect of the active ingredients of various drugs to promote drug absorption and solubility. It is known as a safe carrier for drugs, and excipients also help To give the pills a uniform consistency.
Some people claim that drugs or supplements can be produced without excipients such as magnesium stearate, which is why they are used with more natural alternatives. This may not be the case. In very popular terms: some magnesium stearate alternatives are now being reformulated, using natural excipients such as ascorbyl palmitate, and it makes sense not to misunderstand science. However, these alternatives are not always effective and they also have different physical properties. It is unclear whether magnesium stearate alternatives are possible or needed.

Potential side effects of magnesium stearate
Like other chelated minerals, this mineral has no fixed negative effects because it contains stable neutral compounds composed of minerals and edible acids. On the other hand, overdose of magnesium can impair neuromuscular conduction and, although very rare, can also lead to weakness and diminished reflexes.
Rare things can sometimes happen, and severe toxicities are most common after intravenous infusion over several hours, and can occur after chronic overdose, especially in cases of renal insufficiency. Serious toxicity is reported after acute ingestion, but it is very rare. Still, this report has not reassured everyone.
Suppress T cells
As a key component of the human immune system's attack on pathogens, T cells are not directly affected by magnesium stearate, but are affected by steric acid (the main component of ordinary fillers).
Formaldehyde risk
In a study evaluating common excipients in Japan, plant magnesium stearate was actually found to be a formaldehyde initiator! However, this may not be as scary as it sounds, as many fresh fruits, vegetables and animal products contain formaldehyde. Magnesium stearate produces the smallest amount of formaldehyde per gram of excipient. For example, eating dried mushrooms can produce 406 milligrams of formaldehyde per kilogram!
Create pollution
The World Health Organization released a report outlining how several batches of magnesium stearate were contaminated with potentially harmful chemicals. Because this is an isolated incident, we cannot conclude prematurely that those taking supplements and prescription drugs containing magnesium stearate should be concerned about toxic contamination.
Before resisting all supplements and natural health foods, it's important to consider "dose dependence." In other words, with the exception of intravenous overdose under severe medical conditions, magnesium stearate has only been shown to be harmful in laboratory studies in which rats were forced to feed too high amounts on Earth No one can consume that much.
The study used 40 rats and fed 0%, 5%, 10%, or 20% magnesium stearate on a semi-synthetic diet for 3 months. The 20% group was found to have reduced weight gain, decreased liver weight, and increased iron, kidney stones, and renal calcium deposits. 10% group: liver weight was reduced. 0%-5% group: no side effects were observed, equivalent to less than 2500 mg per kilogram of body weight per day.
This indicates that the amounts of stearic acid and magnesium stearate commonly used in tablets are relatively small. Stearic acid is usually between 0.5% and 10% of the tablet weight, while magnesium stearate is usually between 0.25% and 1.5% of the tablet weight. Therefore, in a 500 mg tablet, the amount of stearic acid may be about 25 mg and the amount of magnesium stearate is about 5 mg.
In fact, magnesium stearate and all its derivatives are cost-effective additives for pharmaceutical and supplement manufacturers. At the same time, however, they pose little or no threat to those who consume it as part of a natural health supplement. All reports claiming that fillers can cause harm are not based on science at all.
I have sorted out the effects and side effects of magnesium stearate in supplements on the human body, and hope to bring a little reference value to everyone. In fact, the author believes that taking too much of anything is always harmful to the body and not beneficial. So it's important to keep this in mind, because if someone is hurt by magnesium stearate, that person needs to consume thousands of capsules or tablets in a day!




